
On June 7th, the official website of the National Medical Products Administration showed that the aspirin enteric coated tablets declared by Nanjing Daoqun Pharmaceutical passed the consistency evaluation and became the first product to be evaluated by Nanjing Daoqun Pharmaceutical.
Aspirin enteric coated tablets are the first evaluated product of Nanjing Daoqun Pharmaceutical. Our company also has another product under review for new registration classification, which is oseltamivir phosphate capsules. In addition, the BE trial of pravastatin sodium tablets is currently underway, and the product has not been reviewed by any company.
经典安全 多效合一
Aspirin (also known as acetylsalicylic acid) is a non steroidal anti-inflammatory drug that has antipyretic, analgesic, anti-inflammatory, and platelet aggregation inhibiting effects. It is suitable for relieving mild or moderate pain such as toothache, headache, neuralgia, and muscle soreness. It is also used for reducing fever in fever diseases such as colds and flu, and for preventing transient ischemic attacks, myocardial infarction, artificial heart valves and venous fistulas, or the formation of blood clots after surgery. In addition, there are also relevant clinical studies that have confirmed that aspirin can prevent and treat diabetes and its complications, senile cataract, stroke and myocardial infarction, antioxidant and malignant tumors, and patients benefit significantly.

[Common name] Aspirin enteric coated tablets
[Specification] 300mg
【 Indications 】
1. Antipyretic and analgesic: It can relieve mild or moderate pain, such as headache, toothache, neuralgia, muscle pain, menstrual pain, arthralgia and migraine. It is also used for fever and sore throat caused by common cold and influenza. This product can only relieve symptoms and cannot treat the causes of pain and fever, so other medications should be used simultaneously to treat the causes.
2. Anti inflammatory and anti rheumatic: commonly used drugs for treating rheumatic fever. After medication, it can relieve fever, improve joint symptoms, and reduce erythrocyte sedimentation rate. However, it cannot remove the basic pathological changes of rheumatic fever, nor can it treat or prevent heart damage and other complications.
3. Arthritis: In addition to rheumatoid arthritis, this product can also improve the symptoms of rheumatoid arthritis, but etiological treatment must be carried out simultaneously. In addition, this product is also used to alleviate symptoms of osteoarthritis, ankylosing spondylitis, gouty arthritis, juvenile arthritis, and skeletal muscle pain in other non rheumatic inflammations. But in recent years, this product has been rarely used for these diseases.
4. Pediatrics is used for the treatment of skin mucosal lymph node syndrome (Kawasaki disease).
5. As an antiplatelet drug, used for acute myocardial infarction.
[Approval Number] National Medical Products Administration Approval Number H32024507
【 Validity 】 18 months
【 Medical Insurance Attributes 】 Class A Basic Medicines under Medical Insurance
【 Production Enterprise 】 Nanjing Baijingyu Pharmaceutical Co., Ltd
【 Listing Authorization Holder 】 Nanjing Daoqun Pharmaceutical R&D Co., Ltd
A century old variety, classic and eternal
Aspirin has a history of 125 years since its launch, and is considered one of the world's three classic drugs along with penicillin and diazepam. Since its launch, aspirin has been extensively used in clinical trials to confirm its pharmacological effects.
According to data from MineNet, in 2022, the sales revenue of terminal aspirin oral regular release dosage forms in Chinese urban public hospitals, county-level public hospitals, urban community centers, and township health centers (referred to as Chinese public medical institutions) exceeded 2.6 billion yuan, with aspirin enteric coated tablets accounting for nearly 98% of sales. From the perspective of manufacturer competition, the original manufacturer Bayer dominates with a market share of over 70%.
In recent years, the sales of terminal aspirin oral regular release dosage forms in public medical institutions in China (unit: billion yuan)
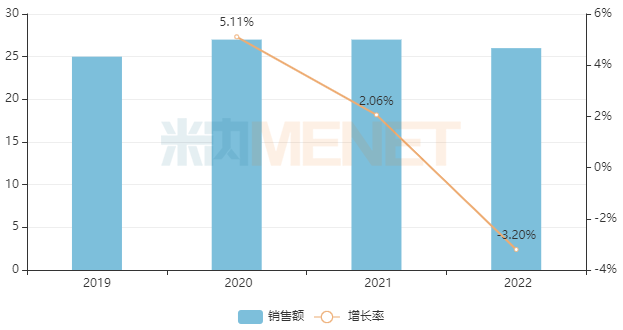
Source: China's public medical institutions' drug terminal competition pattern
At present, there are more than 10 pharmaceutical companies in China that have conducted consistency evaluations of aspirin enteric coated tablets. Among them, products from Lepu Hengyuan Pharmaceutical, Chenxin Pharmaceutical, Chongqing Yaoyou Pharmaceutical, and Nanjing Daoqun Pharmaceutical have been successfully evaluated/deemed to have been evaluated. In addition, products from companies such as Shiyao Group Ouyi Pharmaceutical, Zhejiang Jingxin Pharmaceutical, and Jiangsu Wangao Pharmaceutical are currently under review.
Nanjing Daoqun Pharmaceutical has always adhered to the corporate mission of "assisting medical care and safeguarding health". With the combination of innovation and experience, a strict and standardized quality management system, and professional and efficient research and development, we have successfully obtained this one-time evaluation approval.
In the future, Nanjing Daoqun Pharmaceutical will deepen its cooperation with industry partners as an aggressive "fighter", commit itself to building an excellent Internet medical integration platform, actively promote the development of high-quality domestic drugs, provide more doctors and patients with personalized customized services, and make greater contributions to the industry in this medical journey full of challenges and opportunities.
Data sources: MineNet database, National Medical Products Administration official website, etc